Example: You measure a current of 100 µA in the range 200 µA of the digital voltmeter. Then, the internal shunt is 1 kOhm, across which a voltage of U = R x I = 1000 Ohm x 100 µA = 100 mV now rises.
When measuring the current in usual electronic circuits, this voltage drop may be disregarded. Concerning electrochemical potentials, a drop of 100 mV is nearly enormous: remember that Tafel slopes at ambient temperature may vary by 29 mV per current decade. This example shows drastically that an ammeter for electrochemical measurements should - ideally - have Zero Resitance internal resistance.To achieve this, special "zero - Ohm - ammeters", also called active current sinks (CS), are used. Any potentiostat can be used also as such an active current sink, and it is very simple to do so. Connect the counter electrode to the reference electrode, and use the internal range resistor to define the current range. The current is now directly displayed on the built-in current meter, and a corresponding voltage is fed to the current output terminal.
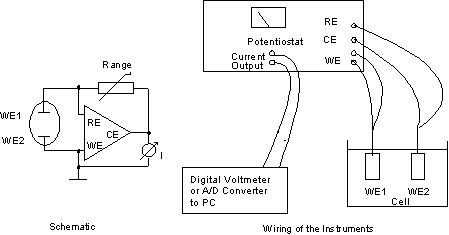
Fig. 4: Potentiostat as Zero - Ohm - Ammeter (Current Sink)
Polarity: The polarity of the current that passes the working electrode is positive if the meter deflects to the right (positive) side. If the internal range resistor does not give the required resolution, you can introduce an external one (see fig. 4, schematic left side). Then the current is measured as the voltages between CE terminal and ground according toI = U / R,
where R is the external range resistor. For R = 1 MOhm, I = U / R = 1 V/µA. A capacitor across the external range resistor will be useful to reduce noise. The time constant of R x C should be in the order of ms for currents down to 1 µA, for lower currents R x C should be in the order of 100 ms up to 1 s. Example: 1 nF x 1000 MOhm = 1 s might be good for measuring currents below 1 nA.
| TOC | Back | Next | |||
| Back Home | |||||